Abstract
Background: Diffuse Large B-cell lymphoma (DLBCL) is common in the elderly patients, Some elderly patients can not tolerate the standard dose of R-CHOP due to age, poor physical condition, and severe complications. The overall response rate of elderly unfit DLBCL patients who received a reduced dose R-CHOP regimen was only 60%. It is extremely important to explore new therapeutic options. Zanubrutinib is a Bruton tyrosine kinase (BTK) inhibitor which was still in an exploratory phase in patients with DLBCL. The efficacy of Zanubrutinib plus Rituximab in elderly unfit Patients with DLBCL is still unknown.
Methods: In the present study, a prospective, single-arm, single-center study was conducted (CHiCTR-2000039342).patients who fulfil the inclusion and exclusion criteria were enrolled. Inclusion criteria: patients aged ≥65 years, and treatment-naive pathologically confirmed with DLBCL, and assessed unfit by Comprehensive Geriatric Assessment(IACA). Unfit patients were defined as follows: i.e. IADL score ≤7, or age >75 years, or CCI score ≥3, or serum albumin <3.4 g/ml. Exclusion criteria: history of malignancy with chemotherapy or radiotherapy before, or serious infectious disease, or bleeding tendency, or hemophilia. Patients enrolled were planned to treat with Zanubrutinib combined with rituximab (Zanubrutinib 160 mg orally twice daily and rituximab 375 mg/m2 day1 iv, every 28 days, ZR regimen), for a total of eight courses. After induction therapy, maintenance treatment with Zanubrutinib 160mg orally twice daily was given until disease progression or unacceptable toxicity. Efficacy was evaluated by PET/CT or enhanced Computed Tomography scan every two to four months, treatment-related adverse effects were evaluated every month. This study was approved by the Ethics Committee of Beijing Hospital, National Center of Gerontology, Beijing, China.
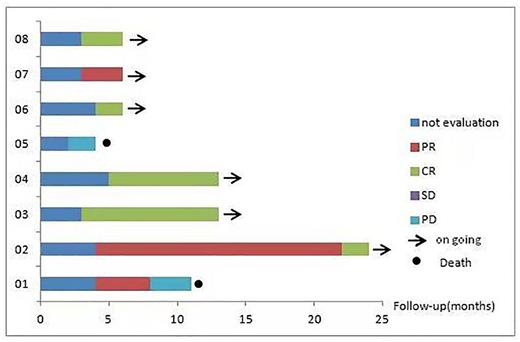
Results: From July 2020 to June 2022, A total of ten elderly unfit DLBCL patients who fulfil the inclusion and exclusion criteria were enrolled(CHiCTR-2000039342).The median age was 86.5 years (80-93 years). The most common comorbidities were cardiovascular disease, hypertension, diabetes mellitus. The female to male ratio was 6:4. Two patients were classified as GCB pathological subtype, Seven patients were diagnosed with stage III-IV. At a median follow up of 9.5 months(2-24months). Eight patients could be evaluated for efficacy. Overall response rate(ORR) was 87.5% (7/8) , five patients (62.5%) achieved complete response(CR). In two patients with GCB pathological subtype, one patient achieved CR, the other one had partial response (PR). In six patients with non-GCB pathological subtype, four patients achieved CR, one had PR, one achieved progression disease(PD) . Median progression-free survival (PFS) was not achieved, one patient had progressing at 8 months, and died due to disease progression, with overall survival (OS) of 11 months. ZR regimen was well-tolerated, three patients had grade 2 urinary tract infections, one had grade 2 respiratory infections, one had grade 1 Petechial hemorrhages, two had grade 2 skin rash, two had acute kidney injury. No grade 3 or higher adverse events were observed. Ten patients had a dose reduction due to toxicity. Two patients had discontinued Zanubrutinib due to adverse events for one to three weeks. In addition, two patients who met exclusion criteria were also off-label use of ZR regimen therapy. They were classified as GCB pathological subtype, and diagnosed with stage III or IV. One patient was 72 years old with IADL score of 6, CCI score of 4, previously diagnosis of lung cancer and renal cancer, treated with radiotherapy and surgery. This patient achieved PR after 2 cycles and had disease progression after 4cycles, with PFS of 4 months. He had grade 2 Petechial hemorrhages or skin rash. The other patient was 81 years old, previously diagnosis of decompensated liver cirrhosis ,with bleeding tendency, IADL score of 2, CCI score of 4.She achieved SD after 2 cycles and had disease progression after 4 cycles, with a PFS of 4 months, and died due to disease progression, with OS of 5 months.
Conclusion: Zanubrutinib in combination with Rituximab may be a well-tolerated and effective regimen in elderly unfit DLBCL patients. Dose reduction may be required for some patients to improve tolerability. A large sample size clinical trial is needed to further validate this conclusion in the future.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Zanubrutinib for the treatment of elderly unfit Patients with previously untreated diffuse large-B cell lymphoma who was not meet the inclusion and exclusion criteria
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal